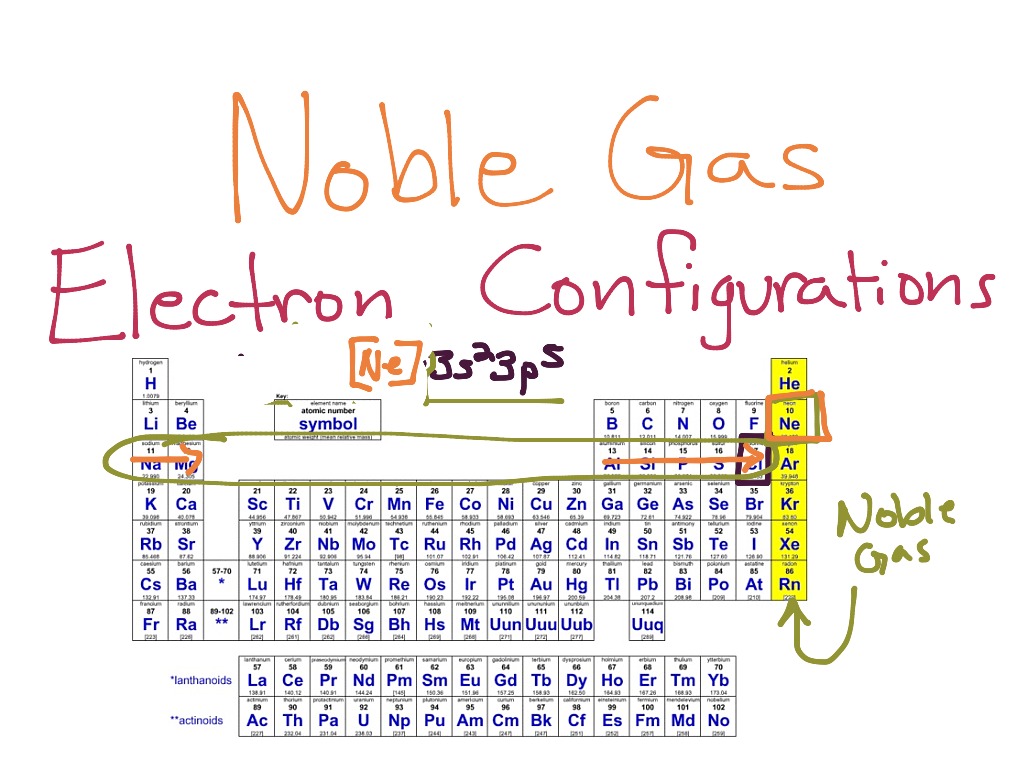
Use Noble-gas Notation to Describe the Electron Configurations of Kr
In a similar fashion strontium has two more electrons than the noble gas krypton which would allow us to write its electrons distribution as Kr5 s 2. Ar4 s 2 c.

Electron Configuration For Neon Ne
The Noble gas notation for Krypton can be written as Kr because it is a Noble gas or Ar4s2 3d10 4p6.
. What element is it. So the shell number for the 1 electron will be 5 since it is after Kr. This is best explained by an example.
Hence the electron configuration for rubidium Rb in noble-gas notation is Kr 5s1. An element has the ground-state electron configuration Kr5s²4d¹⁰5p¹. Kr 5s2 4d2 53 Electron Configuration.
Kr 5s2 4d2 d. The resultant distribution for Pm is. For example the electron configuration of Li would be entered as He2s a.
For atoms with many electrons this notation can become long and therefore an abbreviated notation is used. The noble gas prior to iodine on the periodic table is krypton Kr which has the electron configuration. 2 Pm The atomic number of Pm is 61 so it has 61 protons and 61 electrons.
This is the final electron configuration worksheet. 1 s 2 2 s 2 2 p 5 b. Phosphorus atomic number 15 is as follows.
Written 1S2 2S1 pronounced One-S-Two Two-S-One. Atomic number of Kr 36. Use noble gas shorthand configurations to determine orbital notations.
Use noble-gas notation to describe the electron configurations of the elements represented by the following symbols. So the noble gas before Pm is Xe. Experts are tested by Chegg as specialists in their subject area.
So the 1st 1 which we have to right is krypton which is a noble gas. Abbreviated electron configuration or Noble gas notation describes the electronic configuration of elements based on the last column of elements ie. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 This can be simplified by using the noble gas that covers the.
A deficiency of F can cause increased dental caries and possibly osteoporosis but larger amounts are poisonous. The atomic number of Xe is 54. We review their content and use your feedback to keep the quality high.
The electron configuration of Strontium Sr is. Hf Sc Fe At Ac Zn. Click here to studyprint these flashcards.
Pb Ar4s2 3d10 4p6 Ne3s2 3p3 Kr5s2 4d2 Xe6s2 4f14 5d10 6p2. This problem were asked to write several electron configurations using noble gas notation for four elements. Therefore you have to distribute 61 - 54 7 electrons on the orbitals 6s and 4f.
Krypton - Kr 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 We then replace this section of Zirconiums electron configuration with Kr. Potassium has nineteen electrons one more than the noble gas argon so its configuration could be written as Ar4s 1. Create your own flash cards.
Xe6 s 2 4 f 4 d. Kr is also the last element of the fourth period. Barium In its ground state an atom of an element has two electrons in all orbitals related to.
Who are the experts. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 This is the noble gas core for iodine so the shorthand notation for its electron configuration becomes. Pm is in the row period 6.
But uh well go ahead and use the previous noble gas we have are gone. The Noble gas notation for Krypton can be written as Kr because it is a Noble gas or Ar4s2 3d10 4p6. Kr 5s2 4d2 I hope this was helpful.
What is the noble gas notation of beryllium. Ar 4s2 3d10 4p6 b. 1S2 2S2 2P6 3S2 3P3.
When writing electron configuration we have to follow some rules. Finally the electron configuration is Ar 4s². Express your answer using the noble gas notation.
We have to write the noble-gas notation for. Strontium Kr 5s 2. Ne 3s2 3p3 c.
We have the four this to orbital three d 10 followed by 46. Since s subshell is filled first the electron will go into 5s. Write orbital notations and complete electron configurations for atoms of the following elements.
Kr 4d10 5s2 5p4 Explanation. Answer- The electron configuration of tellurium Te is. Noble Gas Elements Notation.
It is part of some semiconductors and used in various alloys. Elements that have Nobles Gas Configurations. What element is represented by each electron configuration.
An electron will occupy the lowest energy orbital. So only 1 electron needs to be assigned an orbital. The electron configuration can be displayed as a basic electrons equivalent to the noble gas of the previous period.
References Depict the electron configuration for each of the following atoms using noble gas notation. To convert this to the Noble Gas notation we revert back to the Noble Gas from period 4 of the periodic table Krypton.

Electron Configuration Detailed Explanation Filling Of Orbital Representation Of Electronic Configuration Of Atom With Faqs

Noble Gas Notation Electron Configuration Of Ions Aufbau Exceptions Ppt Download

Webelements Periodic Table Krypton Properties Of Free Atoms

Noble Gas Electron Configurations Electron Configuration High School Chemistry Science Showme

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes

How To Write The Electron Configuration For Krypton Kr Youtube
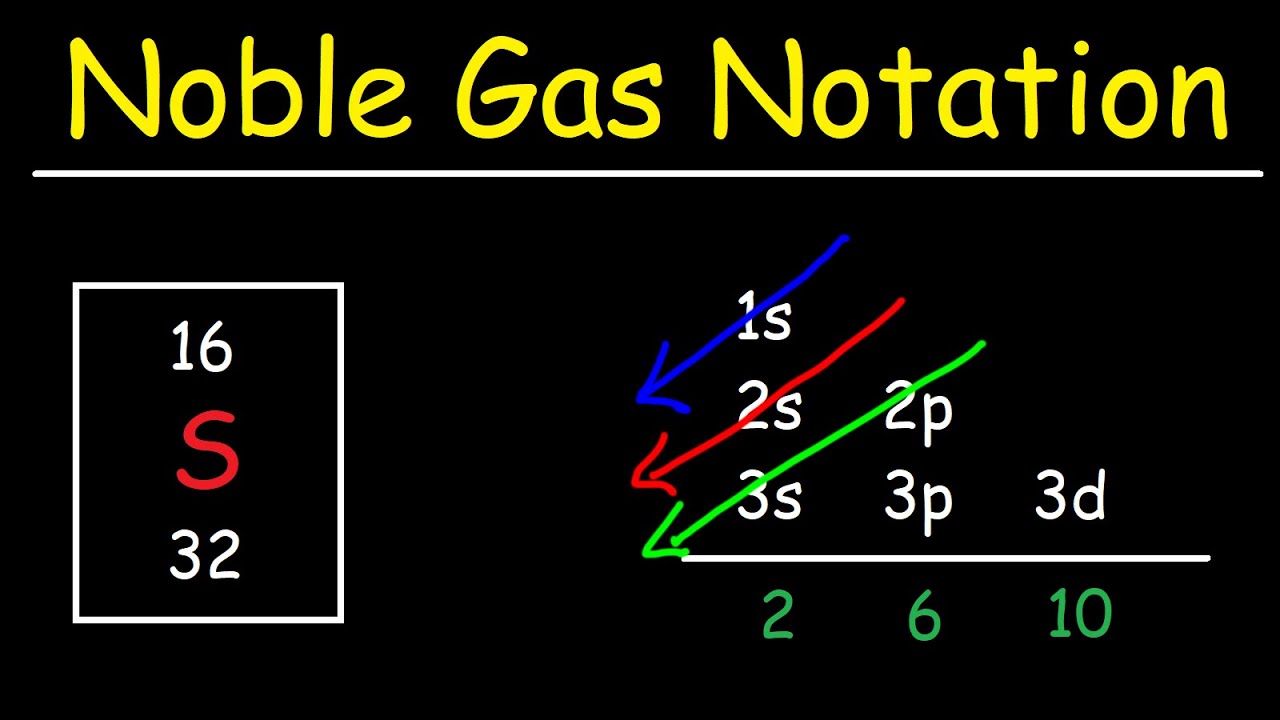
Electron Configuration With Noble Gas Notation Youtube
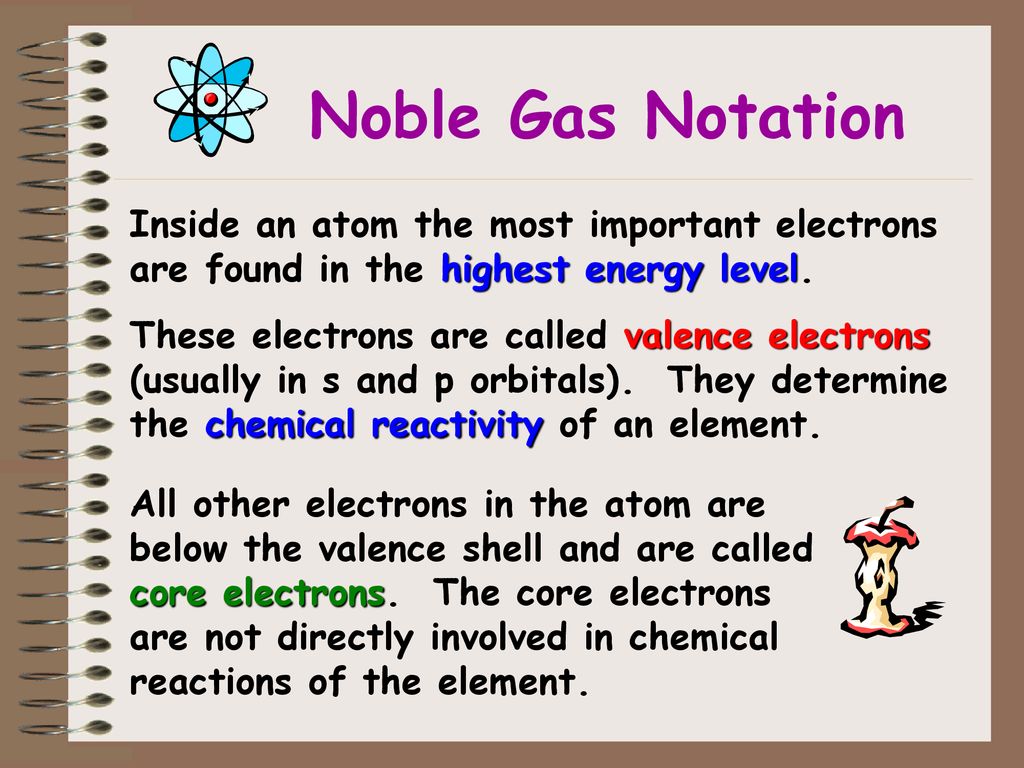
Noble Gas Notation Electron Configuration Of Ions Aufbau Exceptions Ppt Download

Noble Gas Electron Configurations Youtube
What Is The Electron Configuration Of Sc Quora

Electron Configurations And Orbital Notation Diagrams Ppt Download

Electronic Configuration Of Elements And Periodic Table Notes Study Chemistry For Jee Jee
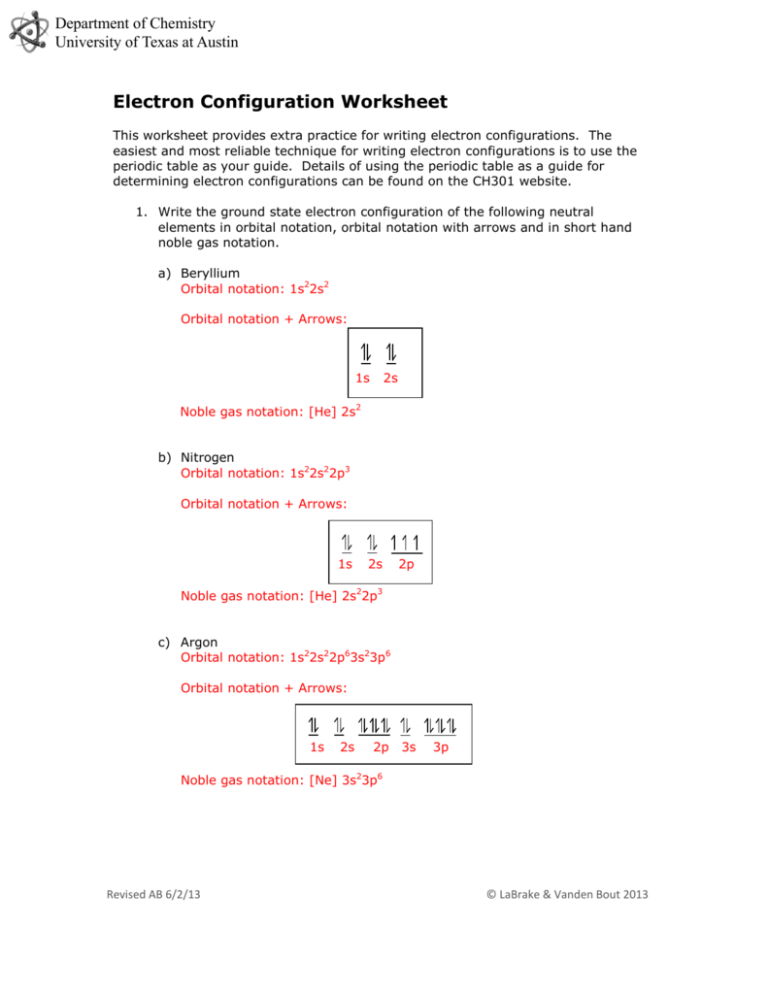
Electron Configuration Worksheet

Krypton Kr Electron Configuration And Orbital Diagram

Question Video Identifying The Condensed Electronic Configuration Of A Potassium Atom Nagwa

Noble Gas Notation Electron Configuration Of Ions Aufbau Exceptions Ppt Download

The Electronic Configuration Of Krypton Is Youtube


Comments
Post a Comment